Acyclic quaternary centers from asymmetric conjugate addition of alkylzirconium reagents to linear trisubstituted enones†
Abstract
Synthetic methods for the selective formation of all carbon quaternary centres in non-cyclic systems are rare. Here we report highly enantioselective Cu-catalytic asymmetric conjugate addition of alkylzirconium species to twelve different acyclic trisubstituted enones. A variety of sp3-hybridized nucleophiles generated by in situ hydrozirconation of alkenes with the Schwartz reagent can be introduced, giving linear products bearing quaternary centres with up to 98% ee. The method is tolerant of several important functional groups and 27 total examples are reported. The method uses a new chiral nonracemic phosphoramidite ligand in a complex with copper triflate as the catalyst. This work allows the straightforward stereocontrolled formation of a valuable structural motif using only a catalytic amount of chiral reagent.
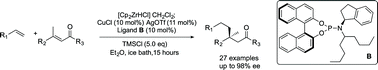

 Please wait while we load your content...
Please wait while we load your content...