Fluorinated antimony(v) derivatives: strong Lewis acidic properties and application to the complexation of formaldehyde in aqueous solutions†
Abstract
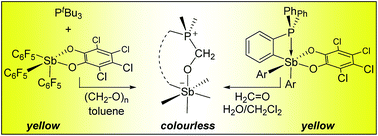
As part of our ongoing studies of water tolerant Lewis acids, we have synthesized and investigated the properties of Sb(C6F5)3(O2C6Cl4), a fluorinated stiborane whose Lewis acidity approaches that of B(C6F5)3. While chloroform solutions of this Lewis acid can be kept open to air or exposed to water for extended periods of time, this new Lewis acid reacts with PtBu3 and paraformaldehyde to form the corresponding formaldehyde adduct tBu3P–CH2–O–Sb(C6F5)3(O2C6Cl4). To test if this reactivity can also be observed with systems that combine the phosphine and the stiborane within the same molecule, we have also prepared o-C6H4(PPh2)(SbAr2(O2C6Cl4)) (Ar = Ph, C6F5). These yellow compounds, which possess an intramolecular P→Sb interaction, are remarkably inert to water but do, nonetheless, react with and accomodate formaldehyde into the P/Sb pocket. In the case of the fluorinated derivative o-C6H4(PPh2)(Sb(C6F5)2(O2C6Cl4)), formaldehyde complexation, which occurs in water/dichloromethane biphasic mixtures, is accompanied by a colourimetric turn-off response thus highlighting the potential that this chemistry holds in the domain of molecular sensing.

 Please wait while we load your content...
Please wait while we load your content...