Preparation of an ion with the highest calculated proton affinity: ortho-diethynylbenzene dianion†
Abstract
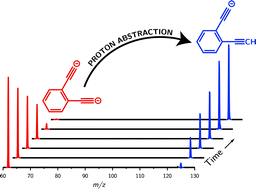
Owing to the increased proton affinity that results from additional negative charges, multiply-charged anions have been proposed as one route to prepare and access a range of new and powerful “superbases”. Paradoxically, while the additional electrons in polyanions increase basicity they serve to diminish the electron binding energy and thus, it had been thought, hinder experimental synthesis. We report the synthesis and isolation of the ortho-diethynylbenzene dianion (ortho-DEB2−) and present observations of this novel species undergoing gas-phase proton-abstraction reactions. Using a theoretical model based on Marcus–Hush theory, we attribute the stability of ortho-DEB2− to the presence of a barrier that prevents spontaneous electron detachment. The proton affinity of 1843 kJ mol−1 calculated for this dianion superbase using high-level quantum chemistry calculations significantly exceeds that of the lithium monoxide anion, the most basic system previously prepared. The ortho-diethynylbenzene dianion is therefore the strongest base that has been experimentally observed to date.


 Please wait while we load your content...
Please wait while we load your content...