Formation and determination of the oxidation products of 5-methylcytosine in RNA†
Abstract
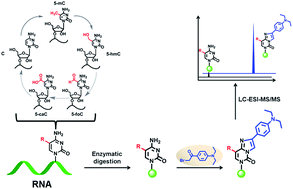
Similar to the reversible epigenetic modifications on DNA, dynamic RNA modifications were recently considered to constitute another realm for biological regulation in the form of “RNA epigenetics”. 5-Methylcytosine (5-mC) has long been known to be present in RNA from all three kingdoms of life. However, the functions of 5-mC in RNA have not been fully understood, especially for the RNA demethylation mechanism. The discovery of 5-hydroxymethylcytosine (5-hmC) in RNA together with our recently reported 5-formylcytosine (5-foC) in RNA indicated that 5-mC in RNA may undergo the same cytosine oxidation demethylation pathway with generating intermediates 5-hmC, 5-foC, and 5-carboxylcytosine (5-caC) by ten–eleven translocation (Tet) proteins as that in DNA. However, endogenous 5-caC in RNA has not been observed so far. In the current study, we established a method using chemical labeling coupled with liquid chromatography-mass spectrometry analysis for the sensitive and simultaneous determination of the oxidative products of 5-mC. Our results demonstrated that the detection sensitivities of 5-mC, 5-hmC, 5-foC and 5-caC in RNA increased by 70–313 folds upon 2-bromo-1-(4-diethylaminophenyl)-ethanone (BDEPE) labeling. Using this method, we discovered the existence of 5-caC in the RNA of mammals. In addition, we found the 5-mC occurs in all RNA species including mRNA, 28S rRNA, 18S rRNA and small RNA (<200 nt). However, 5-hmC, 5-foC and 5-caC mainly occur in mRNA, and barely detected in other types of RNA. Furthermore, we found that the content of 5-hmC in the RNA of human colorectal carcinoma (CRC) and hepatocellular carcinoma (HCC) tissues significantly decreased compared to tumor adjacent normal tissues, suggesting that 5-hmC in RNA may play certain functional roles in the regulation of cancer development and formation.

 Please wait while we load your content...
Please wait while we load your content...