Iminoboronates are efficient intermediates for selective, rapid and reversible N-terminal cysteine functionalisation†
Abstract
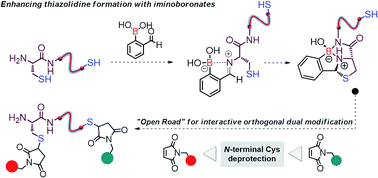
We show that formyl benzeno boronic acids (2FBBA) selectively react with N-terminal cysteines to yield a boronated thiazolidine featuring a B–N bond. The reaction exhibits a very rapid constant rate (2.38 ± 0.23 × 102 M−1 s−1) under mild aqueous conditions (pH 7.4, 23 °C) and tolerates different amino acids at the position adjacent to the N-cysteine. DFT calculations highlighted the diastereoselective nature of this ligation reaction and support the involvement of the proximal boronic acid in the activation of the imine functionality and the stabilisation of the boronated thiazolidine through a chelate effect. The 2FBBA reagent allowed the effective functionalisation of model peptides (C-ovalbumin and a laminin fragment) and the boronated thiazolidine construct was shown to be stable over time, though the reaction was reversible in the presence of benzyl hydroxylamine. The reaction proved to be highly chemoselective, and 2FBBA was used to functionalize the N-terminal cysteine of calcitonin in the presence of a potentially competing in-chain thiol group. This exquisite selectivity profile enabled the dual functionalisation of calcitonin and the interactive orthogonal modification of this peptide when 2FBBA was combined with conventional maleimide chemistry. These results highlight the potential of this methodology to construct complex and well-defined bioconjugates.


 Please wait while we load your content...
Please wait while we load your content...