Nickel-catalyzed C-3 direct arylation of pyridinium ions for the synthesis of 1-azafluorenes†
Abstract
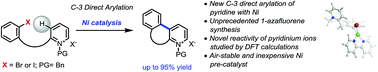
The direct arylation of pyridine substrates using non-precious catalysts is underdeveloped but highly desirable due to its efficiency to access important motifs while being extremely cost-effective. The first nickel-catalyzed C-3 direct arylation of pyridine derivatives to provide a new approach to valuable 1-azafluorene pharmacophore frameworks was developed. This transformation is accomplished using air-stable nickel catalyst precursors combined with phenanthroline ligands and tolerates a variety of substituents. Computational studies suggest facile oxidative addition via the pyridinium form, deprotonation, and a subsequent carbo-nickelation cyclization. Nickel homolysis/recombination permits isomerization to the stereochemical array needed for the final elimination.


 Please wait while we load your content...
Please wait while we load your content...