New insights into the biosynthesis of fosfazinomycin†
Abstract
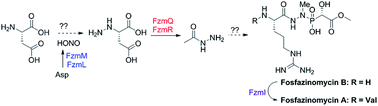
The biosynthetic origin of a unique hydrazide moiety in the phosphonate natural product fosfazinomycin is unknown. This study presents the activities of five proteins encoded in its gene cluster. The flavin-dependent oxygenase FzmM catalyses the oxidation of L-Asp to N-hydroxy-Asp. When FzmL is added, fumarate is produced in addition to nitrous acid. The adenylosuccinate lyase homolog FzmR eliminates acetylhydrazine from N-acetyl-hydrazinosuccinate, which in turn is the product of FzmQ-catalysed acetylation of hydrazinosuccinate. Collectively, these findings suggest a path to N-acetylhydrazine from L-Asp. The incorporation of nitrogen from L-Asp into fosfazinomycin was confirmed by isotope labelling studies. Installation of the N-terminal Val of fosfazinomycin is catalysed by FzmI in a Val-tRNA dependent process.


 Please wait while we load your content...
Please wait while we load your content...