Rhodium catalyzed diastereoselective synthesis of 2,2,3,3-tetrasubstituted indolines from N-sulfonyl-1,2,3-triazoles and ortho-vinylanilines†
Abstract
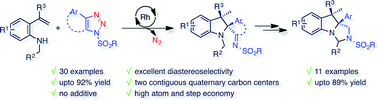
An efficient diastereoselective rhodium catalyzed synthesis of indolines possessing two contiguous tetrasubstituted carbon centers has been achieved with good to excellent yields using ortho-vinylanilines and iminocarbenes derived from N-sulfonyl-1,2,3-triazoles. The reaction affords excellent cis-diastereoselectivity through the initial formation of a N-ylide followed by intramolecular trapping with unactivated alkenes via an ene-type reaction with a well-organized transition state, namely intramolecular carbenylative amination of alkenes. The developed transformation was further extended to the successful synthesis of tricyclic compounds, imidazoindolines, through reduction and hypervalent iodine mediated oxidative cyclization.
- This article is part of the themed collection: Celebrating the Chemical Science in India - Leaders in the Field Symposium

 Please wait while we load your content...
Please wait while we load your content...