Structural switching in self-assembled metal–ligand helicate complexes via ligand-centered reactions†
Abstract
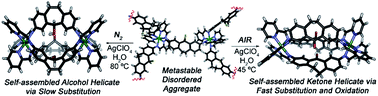
Ligand centered reactions are capable of conferring structural switching between a metastable, self-assembled Fe–iminopyridine aggregate and a stable M2L3 helicate. The reactivity is directed and accelerated by the stability of the final product structure. Under aerobic conditions, both substitution and oxidation occurs at the ligand, exploiting atmospheric oxygen as the oxidant. In the absence of air, reaction occurs more slowly, forming the less stable substitution product. Control ligands show a preference for simple substitution, but the self-assembly directs both substitution and oxidation. The metastable nature of the initial aggregate species is essential for the reaction: while the aggregate is “primed” for reaction, other analogous helicate structures are “locked” by self-assembly, preventing reactivity.

 Please wait while we load your content...
Please wait while we load your content...