ortho-Selective C–H addition of N,N-dimethyl anilines to alkenes by a yttrium catalyst†
Abstract
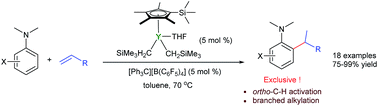
The efficient and selective ortho-alkylation of N,N-dimethyl anilines via C–H addition to alkenes was achieved for the first time using a cationic half-sandwich yttrium catalyst. This protocol constitutes a straightforward and atom-economical route for the synthesis of a new family of tertiary aniline derivatives with branched alkyl substituents, which are otherwise difficult to obtain. DFT calculation studies suggest that the interaction between the yttrium atom and the NMe2 group plays an important role and the intramolecular C–H activation through a σ-bond metathesis pathway is the rate-determining step, which is consistent with the experimental KIE observations.


 Please wait while we load your content...
Please wait while we load your content...