Bismuth oxyhalides: synthesis, structure and photoelectrochemical activity†
Abstract
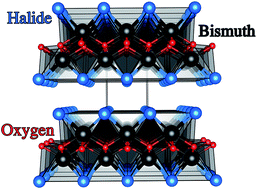
We report the synthesis and photoelectrochemical assessment of phase pure tetragonal matlockite structured BiOX (where X = Cl, Br, I) films. The materials were deposited using aerosol-assisted chemical vapour deposition. The measured optical bandgaps of the oxyhalides, supported by density functional theory calculations, showed a red shift with the increasing size of halide following the binding energy of the anion p-orbitals that form the valence band. Stability and photoelectrochemical studies carried out without a sacrificial electron donor showed the n-type BiOBr film to have the highest photocurrent reported for BiOBr in the literature to date (0.3 mA cm−2 at 1.23 V vs. RHE), indicating it is an excellent candidate for solar fuel production with a very low onset potential of 0.2 V vs. RHE. The high performance was attributed to the preferred growth of the film in the [011] direction, as shown by X-ray diffraction, leading to internal electric fields that minimize charge carrier recombination.
- This article is part of the themed collection: Global Energy Challenges: Materials and Nanotechnology


 Please wait while we load your content...
Please wait while we load your content...