Advances and mechanistic insight on the catalytic Mitsunobu reaction using recyclable azo reagents†
Abstract
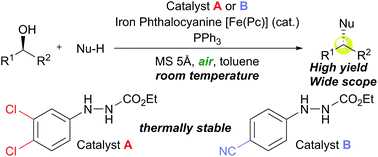
Ethyl 2-arylhydrazinecarboxylates can work as organocatalysts for Mitsunobu reactions because they provide ethyl 2-arylazocarboxylates through aerobic oxidation with a catalytic amount of iron phthalocyanine. First, ethyl 2-(3,4-dichlorophenyl)hydrazinecarboxylate has been identified as a potent catalyst, and the reactivity of the catalytic Mitsunobu reaction was improved through strict optimization of the reaction conditions. Investigation of the catalytic properties of ethyl 2-arylhydrazinecarboxylates and the corresponding azo forms led us to the discovery of a new catalyst, ethyl 2-(4-cyanophenyl)hydrazinecarboxylates, which expanded the scope of substrates. The mechanistic study of the Mitsunobu reaction with these new reagents strongly suggested the formation of betaine intermediates as in typical Mitsunobu reactions. The use of atmospheric oxygen as a sacrificial oxidative agent along with the iron catalyst is convenient and safe from the viewpoint of green chemistry. In addition, thermal analysis of the developed Mitsunobu reagents supports sufficient thermal stability compared with typical azo reagents such as diethyl azodicarboxylate (DEAD). The catalytic system realizes a substantial improvement of the Mitsunobu reaction and will be applicable to practical synthesis.

 Please wait while we load your content...
Please wait while we load your content...