Overcoming naphthoquinone deactivation: rhodium-catalyzed C-5 selective C–H iodination as a gateway to functionalized derivatives†
Abstract
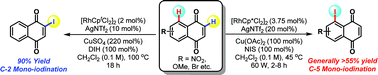
We report a Rh-catalyzed method for the C-5 selective C–H iodination of naphthoquinones and show that complementary C-2 selective processes can be achieved under related conditions. C–C bond forming derivatizations of the C-5 iodinated products provide a gateway to previously inaccessible A-ring analogues. The present study encompasses the first catalytic directed ortho-functionalizations of simple (non-bias) naphthoquinones. The strategic considerations outlined here are likely to be applicable to C–H functionalizations of other weakly coordinating and/or redox sensitive substrates.
- This article is part of the themed collection: SBQ-RSC: Celebrating UK-Brazil collaborations

 Please wait while we load your content...
Please wait while we load your content...