Directed evolution of RebH for catalyst-controlled halogenation of indole C–H bonds†
Abstract
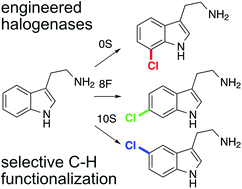
RebH variants capable of chlorinating substituted indoles ortho-, meta-, and para- to the indole nitrogen were evolved by directly screening for altered selectivity on deuterium-substituted probe substrates using mass spectrometry. This systematic approach allowed for rapid accumulation of beneficial mutations using simple adaptive walks and should prove generally useful for altering and optimizing the selectivity of C–H functionalization catalysts. Analysis of the beneficial mutations showed that structure-guided selection of active site residues for targeted mutagenesis can be complicated either by activity/selectivity tradeoffs that reduce the possibility of detecting such mutations or by epistatic effects that actually eliminate the benefits of a mutation in certain contexts. As a corollary to this finding, the precise manner in which the beneficial mutations identified led to the observed changes in RebH selectivity is not clear. Docking simulations suggest that tryptamine binds to these variants as tryptophan does to native halogenases, but structural studies will be required to confirm these models and shed light on how particular mutations impact tryptamine binding. Similar directed evolution efforts on other enzymes or artificial metalloenzymes could enable a wide range of C–H functionalization reactions.

 Please wait while we load your content...
Please wait while we load your content...