Chemoselective nitro reduction and hydroamination using a single iron catalyst†
Abstract
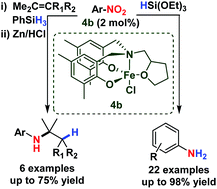
The reduction and reductive addition (formal hydroamination) of functionalised nitroarenes is reported using a simple and bench-stable iron(III) catalyst and silane. The reduction is chemoselective for nitro groups over an array of reactive functionalities (ketone, ester, amide, nitrile, sulfonyl and aryl halide). The high activity of this earth-abundant metal catalyst also facilitates a follow-on reaction in the reductive addition of nitroarenes to alkenes, giving efficient formal hydroamination of olefins under mild conditions. Both reactions offer significant improvements in catalytic activity and chemoselectivity and the utility of these catalysts in facilitating two challenging reactions supports an important mechanistic overlap.

 Please wait while we load your content...
Please wait while we load your content...