Roles of structural plasticity in chaperone HdeA activity are revealed by 19F NMR†
Abstract
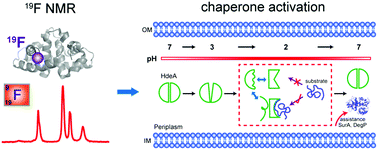
HdeA, a minimal ATP-independent acid chaperone, is crucial for the survival of enteric pathogens as they transit the acidic (pH 1–3) environment of the stomach. Although protein disorder (unfolding) and structural plasticity have been elegantly linked to HdeA function, the details of the linkage are lacking. Here, we apply 19F NMR to reveal the structural transition associated with activation. We find that unfolding is necessary but not sufficient for activation. Multiple conformations are present in the functional state at low pH, but the partially folded conformation is essential for HdeA chaperone activity, and HdeA's intrinsic disulfide bond is required to maintain the partially folded conformation. The results show that both disorder and order are key to function. The ability of 19F NMR to reveal and quantify multiple conformational states makes it a powerful tool for studying other chaperones.

 Please wait while we load your content...
Please wait while we load your content...