Towards pi-extended cycloparaphenylenes as seeds for CNT growth: investigating strain relieving ring-openings and rearrangements†
Abstract
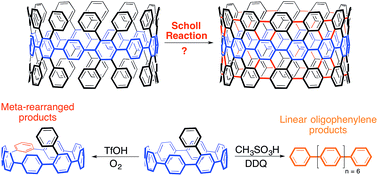
Despite significant multidisciplinary effort over many years, the preparation of uniform carbon nanotubes (CNTs) is still an unsolved problem in the scientific community. This inaccessibility hampers the commercial use of CNTs in electronic devices due to the sensitive connection between their electronic properties and molecular structure. The [n]cycloparaphenylenes ([n]CPPs), the smallest horizontal segment of an armchair CNT, hold great promise as “seeds”, or templates, for the preparation of homogenous batches of CNTs. Initial reports towards this goal, however, suggest that it would be advantageous to pi-extend these structures through traditional organic synthesis before their use in CNT growth. Towards this, several strategies have been reported attempting to utilize the Scholl reaction on aryl-substituted cycloparaphenylenes to yield a small CNT for use as a template for larger tubes. Prominently used in polyaromatic hydrocarbon chemistry, the Scholl reaction has afforded numerous extraordinary targets, such as graphene nanoribbons and graphene propellors. In this work, both experimental and computational studies are provided to unravel the complex cationic rearrangements and ring-openings associated with the Scholl reaction in the context of the cycloparaphenylenes—systems that are thermodynamically and kinetically different from flat graphene fragments. Additionally, this work demonstrates the unique reactivity of cycloparaphenylenes in the context of cationic or radical cationic intermediates, which are common reaction pathways for numerous transformations.

 Please wait while we load your content...
Please wait while we load your content...