Very highly efficient reduction of CO2 to CH4 using metal-free N-doped carbon electrodes†
Abstract
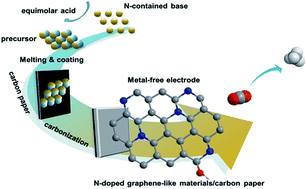
The electrocatalytic reduction of CO2 to energy-rich chemicals is a promising pathway for energy storage and utilization. Herein we report the first work on the electrocatalytic reduction of CO2 to CH4 using metal-free electrodes. It was found that N-doped carbon (graphene-like) material/carbon paper electrodes were very efficient for the electrochemical reaction when using ionic liquids (ILs) as the electrolytes. The faradaic efficiency of CH4 could be as high as 93.5%, which is the highest to date. The current density was about 6 times higher than that of a Cu electrode under similar conditions, which is the well-known effective electrode for the electrocatalytic reduction of CO2 to CH4. Additionally, a trace amount of water in the IL could improve the current density effectively without reducing CH4 selectivity considerably. Our results highlight a new class of low-cost and designable electrocatalysts for synthetic fuel production from CO2.
- This article is part of the themed collection: Global Energy Challenges: Electrochemical Energy

 Please wait while we load your content...
Please wait while we load your content...