Sulfoxide-directed metal-free cross-couplings in the expedient synthesis of benzothiophene-based components of materials†
Abstract
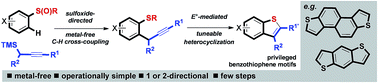
A metal-free approach combining sulfoxide-directed metal-free C–H cross-couplings with tuneable electrophile-mediated heterocyclizations and carbocyclative dimerizations, allows expedient access to benzothiophene-based systems that are components of important materials or are proven organic materials in their own right. As benzothiophene-based materials are typically prepared using Pd-catalyzed cross-coupling processes, our approach allows potential issues of metal cost and supply, and metal-contamination of products, to be avoided.

 Please wait while we load your content...
Please wait while we load your content...