Rhodium-catalyzed asymmetric synthesis of silicon-stereogenic silicon-bridged arylpyridinones†
Abstract
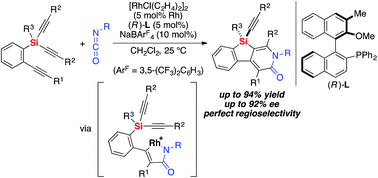
A rhodium-catalyzed regio- and enantioselective synthesis of silicon-stereogenic silicon-bridged arylpyridinones has been developed through [2 + 2 + 2] cycloaddition of silicon-containing prochiral triynes with isocyanates. High yields and enantioselectivities have been achieved by employing an axially chiral monophosphine ligand, and this process could be applied to catalytic asymmetric synthesis of silicon-stereogenic chiral polymers for the first time. The reaction mechanism of the present catalysis has also been experimentally investigated to establish a reasonable catalytic cycle, advancing the mechanistic understanding of the rhodium-catalyzed pyridinone synthesis by [2 + 2 + 2] cycloaddition reactions.
- This article is part of the themed collection: ISACS19: Challenges in Organic Chemistry

 Please wait while we load your content...
Please wait while we load your content...