Mechanistic studies on the addition of hydrogen to iridaepoxide complexes with subsequent elimination of water†
Abstract
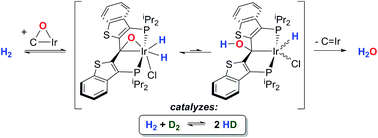
Iridium complexes of the PCsp2P ligand in which the donors are linked by 2,3-benzo[b]thiophene groups engage in the cooperative activation of N2O and the resulting iridaepoxides can be treated with dihydrogen to effect elimination of water and regeneration of the starting iridium complex. The mechanism of the steps in this reaction have been investigated using low temperature NMR investigations that reveal H/D exchange processes that point to a highly reactive kinetic product of hydrogen addition to the iridaepoxide. This intermediate is also involved in the water elimination pathway, and model compounds have been synthesized to provide further evidence for the mechanistic proposals for water elimination. The adaptable donor properties of the PCsp2P ligand framework, particularly the anchoring carbene donor, plays a significant role in the ability of these compounds to mediate the transformation of N2O in this way.
- This article is part of the themed collection: Celebrating our 2018 prize and award winners

 Please wait while we load your content...
Please wait while we load your content...