Zwitterionic and biradicaloid heteroatomic cyclopentane derivatives containing different group 15 elements†
Abstract
The formal cyclopentane-1,3-diyl derivatives [E1(μ-NTer)2({E2C} = NDmp)] (Ter = 2,6-dimesityl-phenyl, Dmp = 2,6-dimethylphenyl) were prepared by 1,1-insertion of CNDmp into the N–E2 bond of [E1(μ-NTer)2E2] (E1 = N, P; E2 = P, As). The insertion does not occur for E1 = E2 = As or E2 = Sb. Dependent on the choice of formal radical centres E, either a biradicaloid or a zwitterion was obtained. The biradicaloid features a P and an As radical center and its biradical character was established by computations as well as characteristic reactivity with respect to the formation of a housane derivative and the activation of molecules bearing multiple bonds, which was demonstrated using the example of PCtBu. In contrast, the formally N,As- and N,P-centered biradicaloids are better regarded as zwitterionic species in accord with computations and diminished reactivity, as neither housane formation nor activation of multiple bonds could be observed.
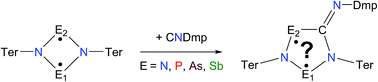
- This article is part of the themed collection: Celebrating the 2017 RSC Prize and Award Winners

 Please wait while we load your content...
Please wait while we load your content...