Enantioselective fluorination of α-branched aldehydes and subsequent conversion to α-hydroxyacetals via stereospecific C–F bond cleavage†
Abstract
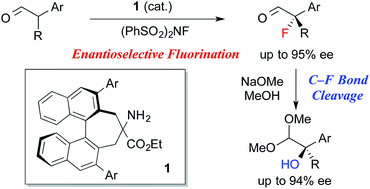
The highly enantioselective fluorination of α-branched aldehydes was achieved using newly developed chiral primary amine catalyst 1. Furthermore, the C–F bond cleavage of the resulting α-fluoroaldehydes proceeded smoothly under alcoholic alkaline conditions to yield the corresponding α-hydroxyacetals in a stereospecific manner. Accordingly, the one-pot conversion of α-branched aldehydes into α-hydroxyacetals was achieved for the first time in high enantioselectivity.

 Please wait while we load your content...
Please wait while we load your content...