Application of online infrared spectroscopy to study the kinetics of precipitation polymerization of acrylic acid in supercritical carbon dioxide†
Abstract
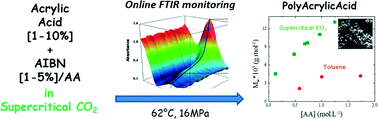
The kinetics of precipitation polymerization of acrylic acid (AA) in supercritical carbon dioxide (scCO2) has been studied using in situ reaction monitoring of the AA/scCO2 phase by Fourier transform infrared (FTIR) spectroscopy. A comparative in situ FTIR study has been performed in toluene. In both solvents, the polymerization kinetics display an induction period in which very little polymerization takes place followed by rapid onset of polymerization to reach a total conversion in about 70 min after the end of the induction period. The rate of polymerization displays an apparent 1.5-order dependence on the monomer concentration and a 0.5-order on the initiator concentration, which is consistent with a mechanism in which the main locus of polymerization is within the particles. The use of scCO2 allows the production of higher molecular weight polyacrylic acid powder than in toluene without any solvent residues.

 Please wait while we load your content...
Please wait while we load your content...