4-Thiazolidinone derivatives: synthesis, antimicrobial, anticancer evaluation and QSAR studies
Abstract
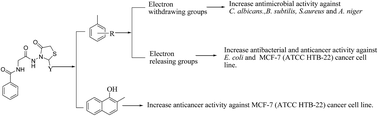
A series of 4-thiazolidinone derivatives (1–18) was synthesized and tested in vitro for its antimicrobial and anticancer potential. Synthesized compounds were found to be 5 more potent antimicrobial agents than anticancer agents. Anticancer screening results indicated that compound 13 (IC50 = 15.18 μM) was the most active anticancer agent and was more potent than the standard drug, carboplatin (IC50 > 100 μM). Antimicrobial activity results indicated that 14 was the most active antimicrobial agent (pMICec = 2.14 μM) and may serve as an important lead for the discovery of novel antimicrobial agents. The QSAR studies indicated that the antibacterial and antifungal activities of the synthesized derivatives against different microbial strains were governed by lipophilic parameter, log P, topological parameter, κα3 and electronic parameters cos E and Nu. E.

 Please wait while we load your content...
Please wait while we load your content...