The interaction between CuO and Al2O3 and the reactivity of copper aluminates below 1000 °C and their implication on the use of the Cu–Al–O system for oxygen storage and production†
Abstract
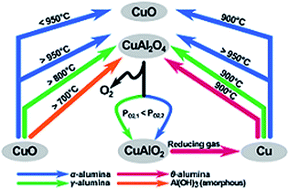
The reversible decomposition of CuO into Cu2O and oxygen at high temperature, typically between 850 and 1000 °C, provides a means of separating pure oxygen from air. In such a process, the oxide generally has to be supported on a refractory oxide, e.g. alumina, to maintain its capacity when cycled many times between CuO and Cu2O. One problem is that if the CuO reacts with the alumina to form CuAl2O4, the latter releases oxygen too slowly to be of practical use so that the capacity for oxygen release of such a carrier falls progressively as more aluminate is formed. However, the reported temperatures at which CuAl2O4 forms are inconsistent so far. This work sets out to investigate the interaction between CuO and different aluminas (and precursors), which are commonly used as support materials, at temperatures between 700 and 1000 °C, as well as some chemical properties of the resulting copper aluminates. It was found that the formation of CuAl2O4 occurred at around 700 °C, 800 °C and 950 °C, when amorphous aluminium hydroxide, γ-alumina, and α-alumina were used as the source of alumina support, respectively. The decomposition of CuAl2O4 in an oxygen-lean environment can lead to the formation of α-alumina as well as γ-alumina, depending on the partial pressure of oxygen. Given that the α-form does not react with CuO around 900 °C, the typical operating temperature for the CuO/Cu2O couple, this observation can be used to partially regenerate CuO from CuAl2O4, for oxygen storage and production at this temperature by decomposing the spinel in a controlled atmosphere to form only α-alumina. However, during the decomposition of CuAl2O4, delafossite CuAlO2 is also formed, limiting the amount of Cu that could be recovered as CuO in a single process cycle.


 Please wait while we load your content...
Please wait while we load your content...