A controlled tandem transformation of conjugated enynones with arenes under superelectrophilic activation leading to aryl-substituted dienones and indenes†
Abstract
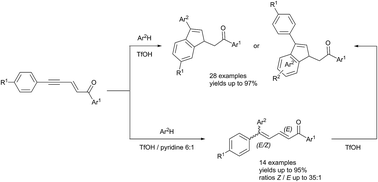
Under superelectrophilic activation in triflic acid (TfOH) and in the presence of arenes, 1,5-diarylpent-2-en-4-yn-1-ones lead to aryl-substituted indenes by addition of arene to the acetylenic bond followed by an intramolecular cyclization in a tandem process, with yields up to 97%. The regiochemistry of product formation is predicted and controlled by varying the aromatic substituents on the starting materials, which makes it possible to obtain diversely substituted indenes. The use of pyridine as a co-solvent of the superacid TfOH allows control of the extent of the tandem transformation and to isolate the aryl-conjugated dienone intermediates before cyclization with high E/Z diastereoselectivities and yields up to 95%. The reaction mechanism is highlighted and involves cationic species that have been observed and characterized by NMR.


 Please wait while we load your content...
Please wait while we load your content...