Polymer–protein hybrid scaffolds as carriers for CORM-3: platforms for the delivery of carbon monoxide (CO)†
Abstract
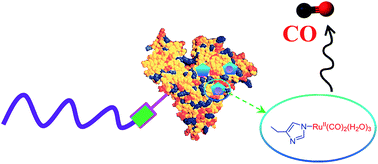
Recognised as a signalling molecule in mammals, carbon monoxide (CO) has a number of beneficial effects, including anti-inflammatory, anti-apoptotic, anti-proliferative and cytoprotective properties, and has been evaluated in clinical trials as a novel therapy. However, the clinical use of CO gas has been hampered due to safety issues and difficulties with delivery to specific sites of action. These challenges have led chemists to explore CO-releasing molecules (CORMs) as an alternative to administration of CO gas. [fac-RuCl(κ2-H2NCH2CO2)(CO)3] (CORM-3), (tricarbonylchloro(glycinato)ruthenium(II)), in particular, has gained increasing attention in biological studies due to its commercial water-soluble CORM and its remarkable biological effects. However, this compound possesses some unfavourable characteristics that have limited its clinical applications, including short CO-releasing half-life, poor stability and low cellular uptake efficiency. These issues can be prevented by incorporating CORM-3 into macromolecular scaffolds. In this study, we present for the first time the use of a polymer–protein hybrid system as a CORM-3 carrier. Firstly, the pyridyl disulfide-functional polymer (2-hydroxy ethyl acrylate) was prepared via Reversible Addition-Fragmentation chain-Transfer (RAFT) polymerisation. The obtained polymer was subsequently immobilised to protein, bovine serum albumin (BSA), via a “grafting to” approach. CORM-3 was then incorporated onto the protein–polymer conjugate BSA–P(HEA) via coordination interaction with histidine residues on BSA. A myoglobin assay demonstrated the CORM-3-functionalised polymer–protein conjugate released CO in a controlled manner in a buffer (pH 7.4) solution, providing slower release compared with CORM-3.

 Please wait while we load your content...
Please wait while we load your content...