A novel porphyrinic photosensitizer based on the molecular complex of meso-tetraphenylporphyrin with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone: higher photocatalytic activity, photooxidative stability and solubility in non-chlorinated solvents†
Abstract
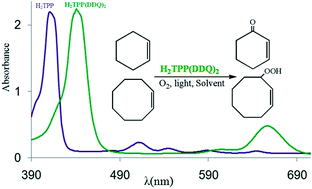
In order to overcome problems associated with the use of porphyrins under photooxidative conditions, the 1 : 2 molecular complex of meso-tetraphenylporphyrin (H2TPP) with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) has been used as a photosensitizer for the aerobic photooxidation of olefins. Under the optimized conditions, H2TPP(DDQ)2 showed a significantly increased catalytic activity and oxidative stability compared to that of the free base porphyrin. Interestingly, 1H NMR and UV-vis studies showed that the DDQ molecules efficiently prevent protonation of H2TPP under acidic condition formed by exposure of chlorinated solvents to the UV-vis light. The photosensitizing ability of the adduct was found to be mainly dependent on the light absorption in the Soret band region and to a much lesser extent on the absorption in the Q(0,0) band region. Furthermore, substantially increased solubility of H2TPP(DDQ)2 in acetonitrile facilitated the use of acetonitrile as a safer solvent than the common chlorinated solvents in photooxidation reactions. Also, the influence of different parameters including the type and power of light source, type of solvent and the molar ratio of H2TPP(DDQ)2 to olefin on the efficiency of the catalyst and the quantum yield of the photochemical reaction (Φ) was investigated. The use of 1,3-diphenylisobenzofuran as the quencher of singlet oxygen and 1,4-benzoquinone as the scavenger of superoxide anion radical revealed the involvement of singlet oxygen as the major reactive oxygen species.


 Please wait while we load your content...
Please wait while we load your content...