Synthesis of fluorinated donepezil by palladium-catalyzed decarboxylative allylation of α-fluoro-β-keto ester with tri-substituted heterocyclic alkene and the self-disproportionation of its enantiomers†
Abstract
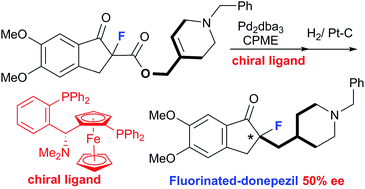
The synthesis of fluorinated donepezil, a promising new therapeutic agent for Alzheimer's disease, was achieved by the palladium-catalyzed decarboxylative allylation (DcA) of an allylic ester having a tri-substituted heterocyclic alkene system as a key step. This strategy is very different from a patented method for the title compound which was successfully extended to the first catalytic asymmetric synthesis of fluorinated donepezil. Fluorinated donepezil showed a noticeable magnitude in the self-disproportionation of enantiomers.

 Please wait while we load your content...
Please wait while we load your content...