Models for the binding channel of wild type and mutant transthyretin with glabridin†
Abstract
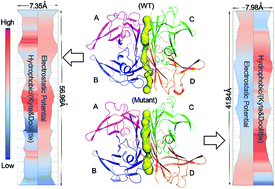
Transthyretin (TTR) is a protein whose aggregation and deposition can lead to human amyloid diseases. V30A TTR is a TTR variant observed in some familial amyloidotic polyneuropathy (FAP) patients. Here we report that V30A TTR exhibits a significantly lower glabridin (Glab) binding energy than exhibited by wild-type TTR (WT TTR) in vitro. To compare changes in structural conformation induced by Glab binding to WT TTR and the V30A mutant, molecular dynamics (MD) simulations were used. Our results indicate that additional high-occupancy hydrogen bonds were observed at the binding interface between the two dimers in V30A TTR, while stabilisation hydrophobic interactions between residues in the mutant AB loop decreased. These results suggest that AC and BD contacts between the two dimers are positioned closer together in the mutant. Our results should provide useful clues to guide future TTR studies.

 Please wait while we load your content...
Please wait while we load your content...