Limonoids with diverse frameworks from the stem bark of Entandrophragma angolense and their bioactivities†
Abstract
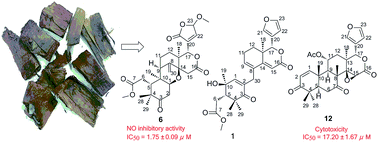
Entangolensins A–P (1–16), sixteen new limonoids with diverse frameworks, were isolated from the stem bark of Entandrophragma angolense. Their planar structures were elucidated on the basis of spectroscopic data (HRMS, 1D/2D NMR), and the absolute configurations of most isolates were determined by the electronic circular dichroism (ECD) exciton chirality method and time-dependent density functional theory (TDDFT)/ECD calculations. Entangolensin A (1) was the first example of C-9/10-seco mexicanolide reported as a natural product. These diverse carbon skeletons, associated in a clear plausible biosynthetic pathway, indicated that the biosynthetic reactions in the title plant were varied and active. Bioactivity screening indicated that compounds 6, 12, 15 and 17 showed moderate cytotoxicities against HepG2 and MCF-7 cell lines, with IC50 values from 13.19 to 36.93 μM; meanwhile, 6, 11 and 17 exhibited significant NO inhibitory activities in LPS-activated RAW 264.7 macrophages, with IC50 values of 1.75, 7.94 and 4.63 μM.

 Please wait while we load your content...
Please wait while we load your content...