DFT calculation of oxygen adsorption on a core-single shell ZnNb catalyst
Abstract
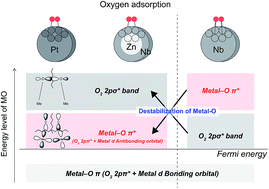
Oxygen adsorption onto Pt, Nb, and core-single shell ZnNb particles is studied using ab initio molecular orbital (MO) calculations to realize their ability for the oxygen reduction reaction (ORR). The calculations demonstrate that the electronic state of oxygen adsorption on core-single shell ZnNb is very close to that on Pt, rather than that on Nb. The energy levels of the oxygen 2pσ* orbital and the antibonding orbital formed by the oxygen 2pπ* and metal d orbitals are different depending on the type of catalyst. In contrast, the energy level of the Nb–O π* (O2 2pπ* + Nb 4d antibonding) orbital of the core-single shell ZnNb is located at a position close to that of Pt. Our finding suggests that the oxygen adsorbed on the core-single shell ZnNb particle can easily be dissociated and desorbed owing to the disappearance of the interaction between the oxygen and Nb subsurface atoms through the replacement of Zn. We expect that core-single shell ZnNb can be utilized as an efficient and cost-effective catalyst for the ORR in a polymer electrolyte fuel cell.

 Please wait while we load your content...
Please wait while we load your content...