Lateral fluoro-substitution and chiral effects on supramolecular liquid crystals containing rod-like and H-bonded bent-core mesogens†
Abstract
The first series of liquid crystalline supramolecular diads C/D containing asymmetric rod-like and H-bonded bent-core mesogens were designed and synthesised, among which supramolecular diad 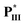 /
/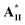 with two chiral centers on both the H-acceptor/H-donor and non-lateral fluoride substitution possessed a wide blue phase (BPI) range of 13.7 °C. According to the molecular modeling, a large biaxial parameter (≥3.2) and an appropriate HTP value (4.2–4.8 μm−1) are the most important factors to stabilize the double twisted cylinder structure (i.e., BPI) in supramolecular diads. Moreover, the bent angles, biaxial parameters and dipole moments of supramolecular diads are mainly affected by the locations and numbers of H-bonds and chiral centers. Not only larger bent angles and biaxial parameters but also smaller dipole moments and HTP values were obtained by removing the lateral fluoride substitution on supramolecular diads to achieve the blue phase of
with two chiral centers on both the H-acceptor/H-donor and non-lateral fluoride substitution possessed a wide blue phase (BPI) range of 13.7 °C. According to the molecular modeling, a large biaxial parameter (≥3.2) and an appropriate HTP value (4.2–4.8 μm−1) are the most important factors to stabilize the double twisted cylinder structure (i.e., BPI) in supramolecular diads. Moreover, the bent angles, biaxial parameters and dipole moments of supramolecular diads are mainly affected by the locations and numbers of H-bonds and chiral centers. Not only larger bent angles and biaxial parameters but also smaller dipole moments and HTP values were obtained by removing the lateral fluoride substitution on supramolecular diads to achieve the blue phase of 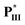 /
/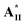 (without lateral fluoride substitution). Finally, the special optimal 1 : 1 molar ratio of supramolecular diad
(without lateral fluoride substitution). Finally, the special optimal 1 : 1 molar ratio of supramolecular diad 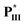 /
/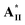 (i.e., H-donor = 50 mol%) with the widest blue phase range is mainly attributed to its rod-like and H-bonded bent-core mesogens, rather than H-donor = 75 mol% in most supramolecular complexes containing linear H-bonded rod-like mesogens.
(i.e., H-donor = 50 mol%) with the widest blue phase range is mainly attributed to its rod-like and H-bonded bent-core mesogens, rather than H-donor = 75 mol% in most supramolecular complexes containing linear H-bonded rod-like mesogens.

 Please wait while we load your content...
Please wait while we load your content...