Phase transition, conduction mechanism and modulus study of KMgPO4 compound
Abstract
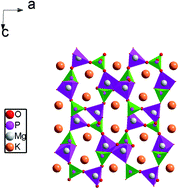
A polycrystalline sample with KMgPO4 stoichiometry was prepared using high-temperature solid-state reaction techniques. X-ray diffraction (XRD) analysis at room temperature shows single-phase formation of the compound with a monoclinic system. The infrared and Raman spectra of this compound were interpreted on the basis of PO43− vibrations. Differential scanning calorimetry (DSC) disclosed two reversible phase transitions of displacive type at T1 = 633 K and T2 = 693 K. Besides, the analysis of Nyquist plots revealed the contribution of three electrically active regions corresponding to the bulk mechanism, distribution of grain boundaries and electrode processes. The variation of ac conductivity versus frequency obeys Jonscher's universal power law. However, increasing frequency levels cause an increase in the ac electrical conductivity. In order to gain an insight into the electric nature of the samples, the Cole–Cole diagrams are also investigated at different temperatures. The temperature dependence of σdc could be described in terms of Arrhenius relation with three activation energies: 0.75 eV in region I (T > T2), 1.19 eV in region II (T1 < T < T2) and 0.95 eV in region III (T < T1). Furthermore, the modulus plots can be characterized by the presence of two peaks associated with the grain and grain boundaries. The near values of activation energies obtained from the equivalent circuit, dc conductivity and modulus studies confirm that the transport is through ion hopping mechanism dominated by the motion of K+ ion along (101).

 Please wait while we load your content...
Please wait while we load your content...