Light-active azaphenalene alkoxyamines: fast and efficient mediators of a photo-induced persistent radical effect†
Abstract
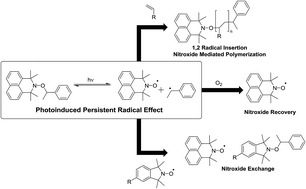
Here we report the first example of an alkoxyamine derived from an azaphenalene nitroxide, which when exposed to UV-light, readily undergoes homolysis to efficiently and cleanly re-form a nitroxide. This process selectively cleaves the C–O bond of the alkoxyamine and occurs orders of magnitude more rapidly than any other alkoxyamine homolysis previously described in the literature. We demonstrate the use of light to generate a persistent radical effect (PRE) and apply this to undertake radical insertion, radical exchange and proof of concept polymerization reactions.


 Please wait while we load your content...
Please wait while we load your content...