Synthesis and characterization of functionalized titania-supported Pd catalyst deriving from new orthopalladated complex of benzophenone imine: catalytic activity in the copper-free Sonogashira cross-coupling reactions at low palladium loadings
Abstract
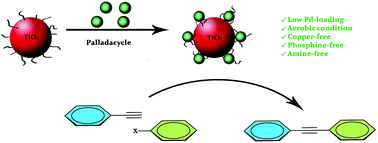
The present work describes the preparation of organically modified TiO2-supported Pd catalyst originated from the new benzophenone imine-derived CN-palladacycle. The heterogeneous organic–inorganic hybrid catalyst system has been characterized by FT-IR, XRD, SEM, EDX, TEM and XPS techniques and exhibited good catalytic activity in the Sonogashira cross-coupling reactions of phenylacetylene with aryl halides under copper-, amine- and phosphine-free conditions, in conjunction with the ultra low catalyst Pd-loading. Significantly, the heterogeneous Pd catalyst allowed the reaction of phenylacetylene with aryl iodides to improve in excellent yields under very mild conditions using green solvent. Finally, the reusability of the supported Pd-complex was investigated by multiple reuses of the supported catalyst in subsequent Sonogashira cross-couplings.

 Please wait while we load your content...
Please wait while we load your content...