Synthesis of 6-hydroxyceramide using ruthenium-catalyzed hydrosilylation–protodesilylation. Unexpected formation of a long periodicity lamellar phase in skin lipid membranes†
Abstract
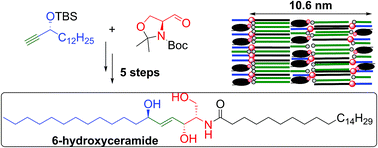
The synthesis of a ceramide with a 6-hydroxysphingosine base, a unique component of the human epidermal barrier, is reported. The key step involves a mild and selective trans-reduction of a triple bond using [Cp*Ru(CH3CN)3]PF6-catalyzed hydrosilylation followed by protodesilylation. The oxidation of sphingosine-based ceramide to 6-hydroxyceramide is also described. X-Ray powder diffraction on the model skin lipid membranes showed that 6-hydroxyceramide promotes the formation of a lamellar phase with 10.6 nm periodicity, which might explain why keratinocytes hydroxylate some ceramides at carbon 6.

 Please wait while we load your content...
Please wait while we load your content...