Noble gas supported B3+ cluster: formation of strong covalent noble gas–boron bonds†
Abstract
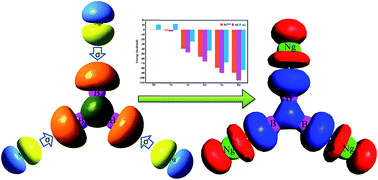
The stability of noble gas (Ng) bound B3+ clusters is assessed via an in silico study, highlighting their structure and the nature of the Ng–B bonds. Ar to Rn atoms are found to form exceptionally strong bonds with B3+ having each Ng–B bond dissociation energy in the range of 15.1–34.8 kcal mol−1 in B3Ng3+ complexes with a gradual increase in moving from Ar to Rn. The computed thermochemical parameters like enthalpy and free energy changes for the Ng dissociation processes from B3Ng3+ also support the stability of Ar to Rn analogues for which the corresponding dissociation processes are endergonic in nature even at room temperature. The covalent nature of the Ng–B bonds is indicated by the localized natural Ng–B bond orbitals and high Wiberg bond indices (0.57–0.78) for Ng–B bonds. Electron density analysis also supports the covalency of these Ng–B bonds where the electron density is accumulated in between Ng and B centres. The orbital interaction energy is the main contributor (ca. 63.0–64.4%) of the total attraction energy in Ng–B bonds. Furthermore, the Ng–B bonding can be explained in terms of a donor–acceptor model where the Ng (HOMO) → B3Ng2+ (LUMO) σ-donation has the major contribution.

 Please wait while we load your content...
Please wait while we load your content...