Synthesis and characterization of 2,2′-bithiophene end-capped dihexyloxy phenylene pentamer and its application in a solution-processed organic ultraviolet photodetector
Abstract
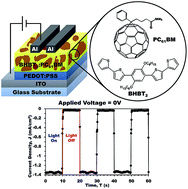
In this work a new solution processable small organic material, namely 2,2′-bithiophene end-capped dihexyloxy phenylene pentamer (BHBT2) was synthesized, characterized and applied in the fabrication of an organic ultraviolet photodetector. The material was synthesized via Williamson etherification, bromination and Suzuki coupling. FTIR and NMR spectroscopies were recorded for BHBT2 along with its optical, thermal and electrochemical properties. Finally, BHBT2 was used as donor material to produce a solution-processed UV photodetector based on a BHBT2 : PC61BM organic active layer. Results showed that in forward biasing, the photodetector exhibited a photovoltaic effect with Jsc = 1.80 mA, Voc = 0.66 V, FF = 0.30 and PCE = 0.98%, while in reverse biasing, the photodetector exhibited a fast, reversible and stable response with the highest detectivity of 1.47 × 109 jones. The realization of efficient UV detection was attributed to the strong absorption of BHBT2 and PC61BM in the UV region. Hence, the BHBT2 pentamer coupled with PC61BM can be considered as a potential material to be applied in a solution-processed organic UV photodetector.

 Please wait while we load your content...
Please wait while we load your content...