Phase-transfer catalyzed enantioselective α-alkylation of α-acyloxymalonates: construction of chiral α-hydroxy quaternary stereogenic centers†
Abstract
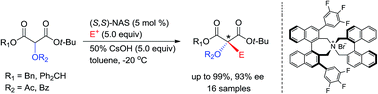
The enantioselective synthesis of α-acyloxy-α-alkylmalonates was developed as an efficient method for producing chiral α-tertiary alcohols, which are potentially valuable intermediates in the synthesis of natural products and pharmaceuticals. The efficient enantioselective α-alkylation of diphenylmethyl tert-butyl α-acyloxymalonates was accomplished via phase-transfer catalysis in the presence of (S,S)-3,4,5-trifluorophenyl-NAS bromide to afford the corresponding α-acyloxy-α-alkylmalonates at high chemical (up to 99%) and optical (up to 93% ee) yields, which could be readily converted to versatile chiral intermediates with a chiral α-tertiary alcohol group.

 Please wait while we load your content...
Please wait while we load your content...