Mixed ionic-electronic conductivity and thermochemical expansion of Ca and Mo co-substituted pyrochlore-type Gd2Ti2O7
Abstract
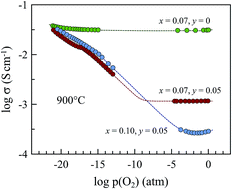
Phase relationships, transport properties and thermomechanical behavior of acceptor- and donor-co-substituted Gd2Ti2O7 were studied for possible application in solid oxide fuel cell anodes. The range of (Gd1−xCax)2(Ti1−yMoy)2O7−δ solid solutions with a cubic pyrochlore-type structure was found to be limited to 0.10 < x < 0.15 and 0.05 < y < 0.10 under oxidizing conditions. No evidence of phase instability of substituted materials was detected in the course of the electrical and thermogravimetric studies down to oxygen partial pressures as low as 10−19 atm at 950 °C. (Gd1−xCax)2(Ti1−yMoy)2O7−δ ceramics possess moderate thermal expansion coefficients compatible with solid electrolytes, (10.5–10.7) × 10−6 K−1 at 25–1100 °C in air, and demonstrate remarkable dimensional stability with nearly zero chemical expansion down to p(O2) ∼ 10−12 atm at 950 °C. Though co-substitution by Mo suppresses oxygen-ionic conduction under oxidizing conditions, reducing oxygen partial pressure increases both ionic and n-type electronic transport. (Gd1−xCax)2(Ti0.95Mo0.05)2O7−δ (x = 0.07–0.10) pyrochlores are mixed conductors under SOFC anode operation conditions, but comparatively low total conductivity limits its applicability as electrode materials.


 Please wait while we load your content...
Please wait while we load your content...