Crystal structure analysis of phycocyanin from chromatically adapted Phormidium rubidum A09DM†
Abstract
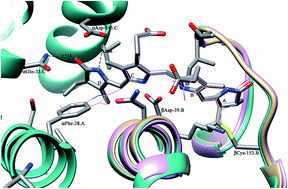
Phycobilisome (PBS), a light harvesting complex of phycobili proteins found in cyanobacteria and red algae, funnels photo-energy to chlorophyll through the network of covalently attached light absorbing chromophores. Phycocyanin, a component protein of PBS, was over-expressed using monochromatic red light in place of white light by chromatically adapted Phormidium rubidum A09DM. The phycocyanin protein, having α- and β-subunits, was isolated and purified in active and chromophorylated form as adjudged by PAGE, MALDI-TOF and spectroscopic analysis. The crystals, obtained using PEG-3350 as a precipitant, belong to the P63 space group with unit cell parameters a = b = 102.40 Å, c = 109.05 Å. The structure has been refined to a crystallographic R factor of 20.7% (Rfree, 25.8%) using X-ray diffraction data extending up to 2.7 Å resolution. The asymmetric unit consists of two αβ monomers. The functional unit [(αβ)3]2 hexamer is generated by the application of crystal symmetry. The overall tertiary structure of α- and β-subunits and hexameric quaternary fold of the Phormidium protein resemble the other reported PC structures, except for the conformation of chromophore attached to βCys-153. The structure and sequence analyses reveal that residues αPhe-28, αGln-33 and αAsp-145 (of α-subunit) are co-evolving and play a key role in determining the conformation of this chromophore. These phycocyanins cluster together in an evolutionary tree and are expected to have evolved later.

 Please wait while we load your content...
Please wait while we load your content...