The investigation of non-noble metal doped mesoporous cobalt oxide catalysts for the water–gas shift reaction†
Abstract
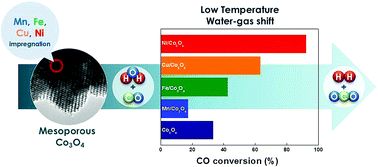
In this study, we report an investigation of the low temperature water–gas shift (LT-WGS) reaction over a series of non-noble metal doped (Me = Mn, Fe, Co, and Ni) mesoporous Co3O4 catalysts. The effect of metal dopants on the structure and reducibility of the mesoporous Co3O4 oxide was examined using X-ray diffraction (XRD), N2-adsorption/desorption isotherm measurements, and H2-temperature programmed reduction (TPR) measurements. Experimental results revealed that among the Me-doped Co3O4 catalysts, Ni/Co3O4 demonstrated the highest catalytic performance (XCO = 93% with 47% H2 yield at 280 °C). The higher activity of the Ni-doped Co3O4 catalyst was mainly due to its smaller crystallite size (8.6 nm) and strong interaction between Co and Ni, which lead to the higher reducibility of Co3O4 compared to the other metal-doped Co3O4. To further optimize the loading of Ni- over the mesoporous Co3O4, a series of Ni(x%)/Co3O4 catalysts were prepared by varying the Ni-loading in the range of 3 to 15 wt%. Among these catalysts, 5 wt% Ni- was found to be the optimum loading, whereas higher Ni-loaded samples (10 and 15 wt%) showed a decrease in catalytic performance and hydrogen yield during the WGS reaction.

 Please wait while we load your content...
Please wait while we load your content...