Synthesis of enantiomerically pure helicene-like mono 1,3-oxazines from 1,1′-binaphthyl-2,2′,7-triol and study of their chiroptical properties†
Abstract
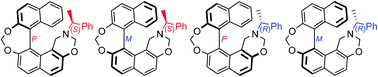
A method was developed to synthesize 1,1′-binaphthyl-2,2′,7-triol by an oxidative cross coupling reaction of 2-hydroxy naphthalene and 2,7-dihydroxy naphthalene. A process was optimized to separate the enantiomers of this compound by making its complex with S-brucine; its absolute configuration was established and it was converted into a series of helicene-like mono 1,3-oxazines. The chiroptical properties of these molecules were investigated and it was established that the helicene-like structural element contributes more to the optical rotation as compared to the stereogenic center.

 Please wait while we load your content...
Please wait while we load your content...