Visible light assisted improved photocatalytic activity of combustion synthesized spongy-ZnO towards dye degradation and bacterial inactivation†
Abstract
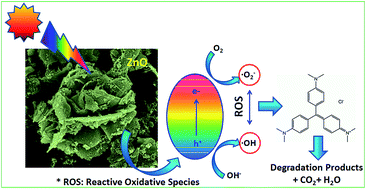
Spongy ZnO photocatalysts were successfully synthesized by a simple, one-step solution combustion technique. The properties such as crystal structure, particle morphology and surface area were optimized through various processing parameters such as oxidizer to fuel ratio, combustion time and the temperature to control crystallinity, phase and yield. The stoichiometric oxidizer to fuel ratio was sufficient to develop a porous and spongy-ZnO nanopowder with a high surface area of 32.6 m2 g−1. The primary particle size of ZnO was found to be ∼60 nm with a band gap of 2.92 eV. The photocatalytic activity of the spongy-ZnO photocatalyst was determined by degrading a model dye, crystal violet under visible light irradiation as well as natural solar light. An antibacterial study was also performed with E. coli as model microorganism under visible light. The E. coli inactivation reaction order was found to be 1 and the rate constant with ZnO was 13.11 ± 0.4 h−1. Spongy-ZnO maintains high photocatalytic activity under solar light irradiation in comparison to P-25 TiO2. The catalytic efficiency was maintained for five cycles under visible light irradiation. Low electron–hole pair recombination with increased efficiency was observed under solar light due to the strong absorption by the photocatalyst under both UV and visible light. The main active species responsible for catalytic degradation are h+ and ˙OH. The probable catalytic mechanism was proposed from the experimentally derived results. The effect of hydrogen peroxide on the photochemical reaction was also studied for dye degradation as well as bacterial inactivation.


 Please wait while we load your content...
Please wait while we load your content...