Structural evolution from CuS nanoflowers to Cu9S5 nanosheets and their applications in environmental pollution removal and photothermal conversion
Abstract
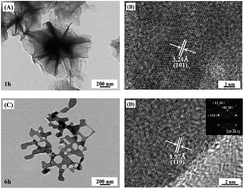
Three-dimensional (3D) CuS nanoflowers and 2D single crystalline Cu9S5 nanosheets with irregular hexagonal holes have been successfully synthesized by easily hydrothermally treating a solution of copper dichloride dihydrates and thiourea at 180 °C for several hours without any surfactants and morphology-controlling agents. Irregular hexagonal holes were widely distributed over the Cu9S5 nanosheets, which could efficiently enlarge the surface area. The UV-vis-NIR absorption spectroscopy showed an obvious increase of the near-IR absorbance. The influence of the reaction time, morphology, and crystal phase on the photocatalysis and photothermal conversion was discussed. The results revealed that CuS nanoflowers displayed the maximum removal value of rhodamine B of 67%. The Cu9S5 nanosheets obtained with the increase reaction time displayed an adsorption and photo-degradation decrease for RhB. The exposure of the aqueous dispersion of the Cu9S5 nanosheets prepared with 6 h (2.0 g L−1) under the irradiation of a 980 nm laser can increase its temperature by 25.1 °C in 600 s, exhibiting a good photothermal conversion performance. The photothermal efficacy increases with the samples obtained from 1 h to 18 h.

 Please wait while we load your content...
Please wait while we load your content...