Effects of chemical structures of polycarboxylic acids on molecular and performance manipulation of hair keratin
Abstract
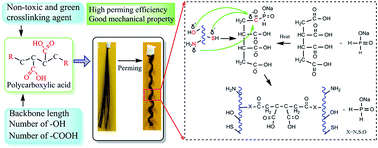
For the first time, we utilized non-toxic polycarboxylic acids in hair perming, and revealed the effects of the chemical structure of polycarboxylic acids on the secondary structure of keratin, and the consequent strength retention and perming effects on the treated hair. Conventional hair perming chemicals were found to be harmful to humans and the environment. However, only in recent years, the adverse effects started to attract public attention. In this study, we discovered that utilizing non-toxic cysteine as a reductant and polycarboxylic acids as crosslinkers could achieve satisfactory hair perming effects and limited loss of hair strength. To quantify the crosslinking efficiency, changes in the amounts of carboxyl groups, amine groups and sulfhydryl groups after different crosslinking treatments were measured via potentiometric and conductometric titrations. The influence of the structures of polycarboxylic acids on the secondary structures of keratin fibers was investigated via wide-angle X-ray diffraction and dynamic mechanical analysis. The α-helix in the secondary structure of hair keratin was partially disrupted, while the amounts of random coil were increased. With an appropriate backbone length, number of carboxyl groups, and number ratio of hydroxyl groups to carboxyl groups, polycarboxylic acids could be optimal for non-toxic hair perming, and have the potential to replace current commercially available harmful hair perming products.

 Please wait while we load your content...
Please wait while we load your content...