Enhancement of the photocatalytic activity of a TiO2/carbon aerogel based on a hydrophilic secondary pore structure†
Abstract
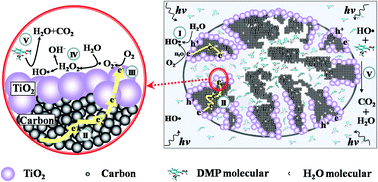
Improving the separation and utilization of electrons and holes in a photocatalytic process is a guarantee for high photocatalytic efficiency. We report a strategy to enhance the photocatalytic performance based on fabrication of a hydrophilic secondary pore structure by incorporating TiO2 into a porous carbon aerogel (CA) with a 9.3 nm pore diameter, where TiO2 resides on both the inner and outer surfaces of CA as evidenced by N2 sorption isotherms and transmission electron microscopy. In such a structure, the spatial separation efficiency of photoelectrons and photoholes is supposed to get enhanced with interface electrons transferring into the inner surface of the pores via conductive CA. As a result, HO˙ formation can be promoted in the confined inter surface of the hydrophilic secondary channel through O2 reduction with the participation of photoelectrons and H2O. And the remaining photoholes on the outer surface can oxidize water to generate HO˙ as well. In contrast, TiO2 is mainly dispersed on the outer surface of CA as small pore diameters of 3.4 and 4.3 nm; as a result, only uncombined photoholes on the outer surface contribute to HO˙ generation via the water oxidation route. In line with this understanding, TiO2/CA (9.3 nm) shows the largest amount of HO˙ and thereby the highest efficiency of dimethyl phthalate degradation, as respectively evidenced by the electron paramagnetic resonance spectroscopy and the photocatalytic degradation test. These findings unveil the contribution of the surface/interface synergy effect on the separation and utilization of electrons and holes in photocatalytic process, and provide a potential strategy to enhance the photocatalytic performance.

 Please wait while we load your content...
Please wait while we load your content...