Corrosion of aluminium alloy 1100 in post-LOCA solutions of a nuclear reactor
Abstract
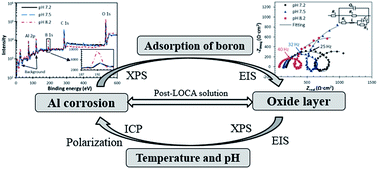
The corrosion and release of aluminum alloy 1100 in characteristic solutions in the containment sump of a nuclear reactor after a loss-of-coolant accident has been studied by an integral approach of electrochemical characterization and solution analysis by inductively coupled plasma (ICP) techniques. Various temperatures and pHs for a reactor in post-LOCA conditions are investigated to identify the effects of the temperature and solution composition on the corrosion. It was found that the oxygen reduction dominants the cathodic reaction below 55 °C, while water reduction becomes more pronounced at a higher temperature. The adsorption of boron on aluminum was identified by electrochemical impedance spectroscopy (EIS) and X-ray photoelectron spectroscopy (XPS). The boron adsorption decreased the thickness of the passive film by increasing the aluminum hydroxide solubilities, resulting in a very thin (<1.5 nm) passive film in the high boron concentrated post-LOCA solutions. The aluminium corrosion rate and passive film resistance obey an Arrhenius-type correlation with temperature in post-LOCA solutions. The slopes of the logarithmic release rate of aluminium in post-LOCA solutions against the pH and the reciprocal temperature are found to keep constant in solutions with a pH between pH 4–12, and a temperature range of 25 to 130 °C.

 Please wait while we load your content...
Please wait while we load your content...